Spent mushroom compost as casing soil with Mohammad Mirzadeh
Mohammad Mirzadeh discusses his experience recycling spent compost for use in casing materials
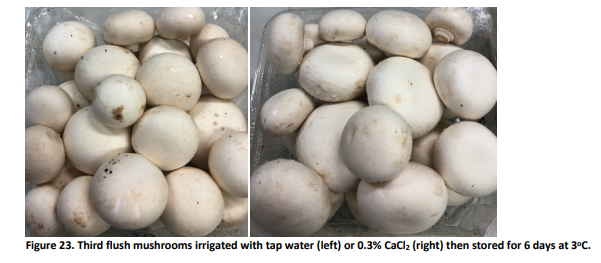
MU19005 - New innovations to improve mushroom whiteness shelf life
Key research provider: Applied Horticultural Research
During 2020, this investment investigated new innovations to improve and maintain mushroom whiteness, looking at both pre- and post-harvest factors. A grower-focused Best Bets Guide was produced that outlines the most effective technologies, techniques and strategies that mushroom growers can use on-farm to improve and maintain mushroom whiteness, with information to improve handling and management through the supply chain as well.
Presenting clean, white mushrooms to consumers at retail is a proven method of increasing sales. Conversely, browning mushrooms lack freshness, appearing damaged or near the end of storage life. Improving and maintaining whiteness has the potential to boost sales and reduce waste, and ultimately increase the profitability of the mushroom sector.
The project team conducted a detailed desktop study on mushroom whiteness. Peer reviewed literature on mushroom quality has increased immensely in the last few years, with substantial information about the cellular and metabolic process involved in the browning of mushrooms, along with a wide range of methods to slow this process.
As well as detailing the causes of browning, the project’s study thoroughly reviewed pre-harvest, harvest and post-harvest strategies to improve whiteness at harvest and retain colour through the supply chain.
Promising areas requiring further work to achieve commercialisation have been identified, along with proven methods that industry could adopt right now.
Project outputs
Access the Best Bets Guide on the industry resource website AGORA.
Read this article about the project’s results in the Summer 2020 edition of Australian Mushrooms Journal.
Detergent & farm sanitation
Starting a new crop in a room free of pests and pathogens is the most potent and cost-eff effective tool for disease management on the mushroom farm, but it doesn’t come easily. Grow room sanitation has two distinct stages – cleaning and disinfection.
Too often, we expect our disinfectants to do the heavy lifting and presume they will eradicate the majority of the pathogens. After all, their job is to kill pathogens. But the truth is, it is the cleaning process before disinfection that removes the majority of the microbes and pathogens from a heavily soiled environment and not the disinfectant.
This article was originally produced for the Australian Mushroom Journal issue 2 2021
Australian Mushroom Industry - Can nitrogen be better managed in compost production
Button mushrooms contain high levels of minerals, vitamins and antioxidants, but are also an excellent source of protein. With 19 - 35% protein per gram of dry weight, they contain more protein than rice (7.3%), wheat (13 %) or milk (25%), and the high content of essential amino acids also means that button mushroom proteins are 90-98% as nutritious as most meat protein.
The nitrogen required to build these proteins comes from the compost, partly from raw materials such as manure, and partly from supplements added later in the process. The carbon:nitrogen ratio in the starting compost mix is usually set to between 30:1 and 35:1, which is optimal for growth of the microbes that convert the straw into productive compost, but only about 12-15% of this nitrogen finishes up in the mushroom crop that goes to market.
This webinar will discuss how nitrogen is transformed into mushroom protein during composting and cropping, where losses occur, and how changes in starting materials or composting processes might be used to increase compost productivity and the nutritional value of the mushroom crop.
Climate change and the Australian mushroom industry – risk, adaptation and opportunities
Climate change and its impact are back in the world debate. Although mushrooms would seem less affected by climate change than other crops – being protected from extremes of weather, the industry remains vulnerable to climate-related risks.
There are also climate-related opportunities to reduce costs and improve the environmental performance of the Australian mushroom industry. E.g., onsite energy generation, new growing substrates and water recycling. Adopting new technologies can reduce costs, sidestep production limitations, and enhance the industry's “green” image.
Join Dr Jenny Ekman, Liam Southam-Rogers and Adam Goldwater who will explain why what’s good for the environment, can also be good for your business when they summarise the outcomes of the levy funded project “Understanding and managing the impacts of climate change on Australian mushroom production”.
Lecanicillium fungicola– Dry Bubble disease
Dry Bubble is the common name given to a serious fungal disease affecting cultivated Agaricus bisporus crops. It is caused by the soil-borne mycoparasites Lecanicillium fungicola var fungicola (syn: Verticillium fungicola; Verticillium malthousei) which is found in Europe and Lecanicillium fungicola var aleophilum which is more common in North American Agaricus crops, including Agaricus bitorquis. Dry Bubble is consistently the most significant problem facing growers wherever button mushrooms are grown, including Australia.
Lecanicillium fungicola was first identified as a mushroom pathogen in France in 1892 and was described as the causal agent of ‘La mole’ disease, the French term for what we now refer to as Dry Bubble. But despite more than 130 years since its first appearance, Dry Bubble continues to have a detrimental impact on mushroom production. The disease causes significant losses estimated at 2-4% of total revenue annually and poor control of the disease may result in losses approaching 20-25% or more, while uncontrolled disease can result in farm closure.
Mushroom Virus Disease - Biology and Epidemiology
Viruses are essentially non-living particles that need to exist inside a host to be able to survive and multiply. Two virus diseases of Agaricus bisporus are known:
La France disease, first recorded in 1948 in the United States.
Mushroom Virus X (MVX) disease, first recorded in the United Kingdom in 1996.
… If virus disease is not identified early and management strategies implemented, virus infected mushroom spores will accumulate on farm, creating disease reservoirs.
This Article was originally produced for the Australian Mushroom Journal Issue 4 2020.
Mushroom Virus - Frustrating and costly
This Article was originally produced for the Australian Mushroom Journal Issue 4 2020.
Reviewing the factors that improve mushroom whiteness
For mushrooms, whiteness signals quality. It may also be assumed to indicate storage life, flavour and freshness. Presenting clean, white mushrooms to consumers is a proven way to increase sales. Conversely, browning on mushrooms is definitely a negative. Browning may be due to disease, bruising, dehydration or simply age and senescence.
Project MU19005 has reviewed the factors that improve mushroom whiteness, from the time compost and casing are prepared through to harvest and packing. The result is a combination of strategies growers can use right now, techniques that are close to commercialising and advances to watch into the future.
Dr Jenny Ekman will summarise the results from this review and discuss some of the “Best Bets” growers can use to improve mushroom whiteness.
Recycled organics as an alternative to peat in mushroom casing
The Australian mushroom industry uses approximately 25,000 tonnes of peat casing every year. Mostly imported from Europe or Canada at a cost of $300 per tonne, peat is both an expensive and limited resource.
Compost made from recycled organics is locally available and cheaper than peat.
Join Adam Goldwater from Applied Horticultural Research for a webinar where he will present the results of the recent trials of commercially viable white mushroom crops cased with blends of composted recycled organics and peat.
This is a Waste Less Recycle More initiative funded from the waste levy.
Getting the best from your cookout
As mushroom crops mature, pest and pathogen levels increase so that by the end of the crop, the pathogen population reaches its maximum (Fletcher & Gaze 2008). Effective crop termination is essential to reduce the pathogen population, allowing the next crop to ‘start clean’ and to break the cycle of diseases, such as Dry Bubble, which are perpetuated by continual on-farm re-infection.
By far the most effective termination procedure is cookout in situ, where the crop is treated undisturbed in the grow room with steam. An effective cookout prevents contamination of subsequent and adjacent crops which occurs when spent substrate contaminated with viable pathogens, pests and their larvae is removed from a grow room (Beyer 2018).
Cookout must kill pests and pathogens within the compost and netting on shelf farms and within the compost and tray timbers on tray farms. Cookout must also kill Agaricus mycelium and spores within the compost and tray timbers to prevent spread of virus diseases.
Cladobotryum spp. – Cobweb disease
Cobweb is the common name given to a fungal disease affecting Agaricus bisporus crops in mushroom growing regions worldwide.
It is caused by species of the genus Cladobotryum (formerly Dactylium), primarily Cladobotryum mycophilum and Cladobotryum dendroides. The pathogen grows rapidly over the casing surface and colonizes mushrooms at all stages of development with a white aerial mycelium, causing a destructive soft rot. Cladobotryum sporulates heavily and the spores are easily spread around the farm causing secondary infections. Spores landing on mushroom caps incite browning, causing loss of quality.
Until the early 1990s, cobweb outbreaks had little impact and were easily controlled by available fungicides and routine hygiene practices. But during the early 1990s, the incidence and severity of cobweb on British mushroom farms increased and in 1994/95 the disease reached epidemic proportions, regularly causing up to 40% crop loss.
Nematodes: A fly-in-fly-out pest of mushrooms crops
Long distance dispersal of nematodes occurs by a process called ‘phoresy’, the transport of a small animal by a larger animal.
In mushroom grow rooms, nematodes attach to Sciarid flies and are transported to fresh mushroom beds to establish new colonies and new infections.
This is further evidence demonstrating why effective fly control is highly significant in successful management of mushroom diseases and pests.
This article was originally produced for the Australian Mushrooms Journal 2020 Issue 1
Penicillium hermansii – Smoky mould
Smoky mould is a destructive compost infection first recognized in the Netherlands nearly 30 years ago. Despite being identified as various Penicillium species over the years, the true identity of the causal organism has only recently been confirmed through molecular analysis as Penicillium hermansii.
Penicillium species produce long chains of very small, lightweight, dry spores which are around 0.002mm in diameter (Fig. 1) and become airborne very easily. A single Penicillium colony (Fig. 2) will produce 400,000,000 spores per day. Although P. hermansii is very slow growing, it is problematic because of the large number of spores it produces and its short generation time. Penicillium hermansii will sporulate only three or four days after infection, producing thousands of daughter colonies which in turn sporulate rapidly.
Action points to control Sciarid & Phorid flies
Flies are effective vectors of disease because the sticky disease spores attach themselves to the flies legs and the flies transport them from crop to crop.
Sciarid and phorid flies can breed in bushland, ‘waste’ or ‘spent’ compost in the farm environment and, most efficiently of all, in growing rooms.
The odour associated with the Phase 3 compost arriving on a farm either in bulk or blocks acts like a strong magnet to attract flies to the new crop.
The fact that a female sciarid can produce around 100 offspring and a female phorid can produce around 50 offspring means that new crops need to be strongly protected from invasion by adults and a holistic and integrated approach to fly control is needed.
Sampling Methods for Mushroom Pest and Disease Testing
This video describes how to collect samples for disease testing using molecular testing techniques.
This video was produced as part of the pest and disease management and research services (MU16003) project.
It was funded by Hort Innovation using the Mushroom Industry research and development levy and contributions from the Australian Government.
Fact sheet: Syzygites megalocarpus – Troll doll
Troll doll is caused by Syzygites megalocarpus a Zygomycete which is ubiquitous in nature, colonizing a diverse variety of dead or moribund fleshy mushrooms. Syzygites (pronounced “size‐a‐guy‐tees”) was initially observed on cultivated mushrooms between 2004 and 2007 in crops of Agaricus blazei (Sun mushroom) in Brazil.
It was first recorded in Pennsylvania in August 2011 and has since become widespread on commercial beds throughout North America.
Confined initially to late flushes of brown portobello strains of Agaricus bisporus, Syzygites has since been observed on earlier flushes and on white strains of Agaricus bisporus. Due to the mould’s tolerance to low temperatures, it has also been observed in postharvest packaged product, the mould appearing while on the store shelf.